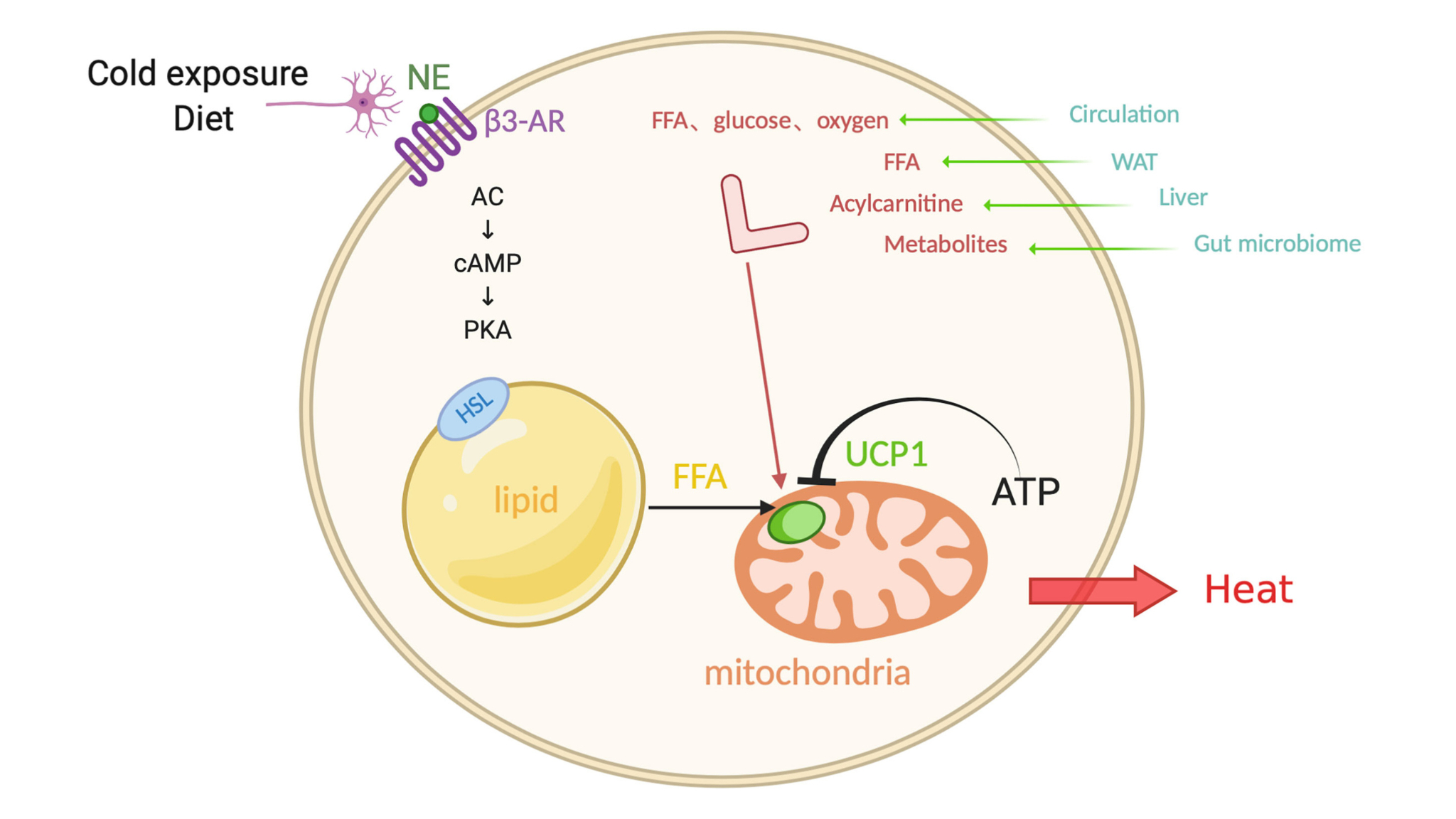
Brown adipocytes stand out from other fat cells because they generate heat through cell signaling technology UCP1. These specialized cells produce heat by expressing uncoupling protein-1 (UCP1), which lives in the mitochondrial inner membrane.
Research shows that brown adipose tissue activity correlates positively with energy expenditure. This activity helps reduce the risk of metabolic syndrome and cardiometabolic diseases. Cell Signaling Technology’s research has improved our understanding of UCP1’s vital role in nonshivering thermogenesis. Studies reveal that UCP1 knockout mice become obese under thermoneutral conditions and cannot tolerate cold.
In this piece, let’s take a closer look at the molecular mechanisms that activate UCP1 and the various detection methods available. We’ll explore its potential therapeutic applications for treating obesity-related disorders. Cell Signaling Technology’s verified protocols and technical support resources offer valuable tools to study this significant protein.
Cell Signaling Technology UCP1 Methods Detection
Scientists must use exact methods and proven protocols to detect UCP1 protein accurately. Cell signaling technology gives us three main ways to detect and analyze UCP1.
Western Blot Protocols and Optimization
The high lipid content in adipose tissue means you need careful sample preparation for Western blot analysis. Scientists use the acetone precipitation method to remove leftover lipids, which results in clearer UCP1 protein bands without any contamination. Mouse brown adipose tissue analysis works best with a 1:2,000 dilution of UCP1 Polyclonal antibody and HRP Goat Anti-Rabbit IgG at 1:10,000.
Here’s the quickest way to get great Western blot results:
- Use RIPA or adipocyte lysis buffer to prepare samples
- Extract protein with acetone precipitation
- Load 25 µg protein per lane
- Block with 3% nonfat dry milk in TBST
- Detect with ECL using 180-second exposure
Immunofluorescence Techniques
Immunofluorescence analysis helps us learn about UCP1’s distribution in brown adipose cells. The technique shows UCP1 appearing in punctate structures between lipid droplets. You’ll get the best visualization by incubating samples with UCP1 Polyclonal antibody at 1:100 dilution and DAPI nuclear staining. High-pressure antigen retrieval with 10 mM citrate buffer (pH 6.0) boosts staining quality significantly.
Flow Cytometry Applications
Flow cytometry is a chance to analyze UCP1 in a unique way. It lets you examine thousands of cells individually. This method spots UCP1-positive adipocytes, which make up approximately 18% of adipocytes in scWAT after β3-adrenergic agonist treatment. On top of that, it measures cell size through forward scatter (FSC) and granularity through side scatter (SSC).
Latest flow cytometers can handle adipocytes with their 150-250 μm internal fluidics diameter at pressures between 5-10 psi. This setup lets you analyze adipocyte populations while keeping cells intact. The method works especially well to examine brown adipocyte differentiation and measure UCP1-expressing cells that respond to various stimuli.
Molecular Mechanisms of Cell Signaling Technology UCP1 Activation
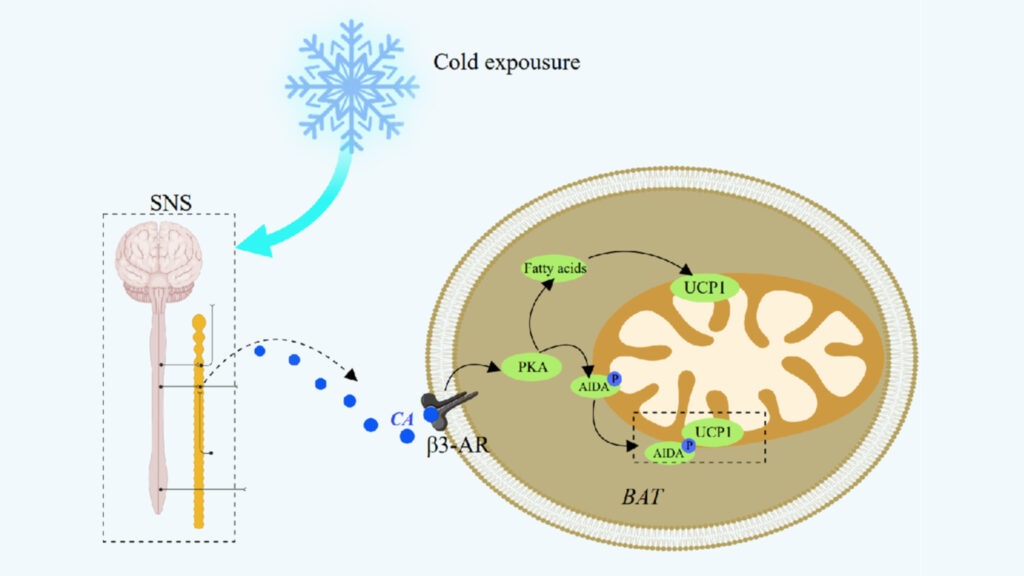
The sympathetic nervous system starts UCP1 activation through a complex cascade of molecular events. Cold exposure triggers norepinephrine release that binds to β3-adrenergic receptors on brown adipocytes.
Beta-Adrenergic Signaling Cascade
Cold stimulation causes β3-adrenergic receptor activation and sets off a series of intracellular events. The process starts when Gαs protein dissociates from stimulated receptors and activates adenylate cyclase. Brown adipose tissue responds to environmental temperature changes by increasing sympathetic nerve activity.
cAMP-Dependent Pathways
Receptor activation substantially increases cellular cAMP levels and triggers both acute and long-term responses. The immediate effects include:
- Activation of hormone-sensitive lipase
- Phosphorylation of perilipin protein
- Release of fatty acids from triglyceride stores
- Direct activation of UCP1-mediated thermogenesis
Cellular cAMP activates protein kinase A, which phosphorylates hormone-sensitive lipase and perilipin. This process guides lipolysis of triglyceride stores and releases free fatty acids into the cytosol. These fatty acids serve as fuel for mitochondrial oxidation and direct activators of UCP1.
Protein Kinase A Regulation
PKA arranges multiple aspects of UCP1 activation and regulation. PKA’s activation triggers the p38 MAP kinase pathway. This pathway is a vital part of UCP1 gene’s transcriptional control through its complex enhancer region. The enhancer region contains binding sites for several nuclear receptor transcription factors, including PPARγ, RXR, RAR, and thyroid hormone receptor.
PKA’s signaling pathway affects UCP1 activity through various mechanisms. Brown adipocytes’ PKA activation creates changes in both acute responses and transcriptional regulation. Rapid phosphorylation of multiple targets results in increased UCP1 activity. These pathways help UCP1 activation increase fatty acid oxidation to compensate for respiratory inefficiency and decreased mitochondrial membrane potential.
Technical Support for Cell Signaling Technology UCP1 Research
UCP1 research success depends on solid technical support and proven protocols. Cell Signaling Technology offers complete resources that help researchers get accurate results in UCP1 studies.
Cell Signaling Technology UCP1: Antibody Selection Guidelines
The right antibody choice needs proof of specificity and validation data. Researchers should look beyond manufacturer claims and check that antibodies show no cross-reactivity with UCP2 or UCP3 proteins. Quality UCP1 antibodies detect natural levels of total UCP1 protein and target specific spots like Pro152 of human UCP1 protein.
Troubleshooting Common Issues
Fatty acid interference creates challenges in UCP1 detection. These protocol adjustments will help:
- Use 0.5-1% fatty acid-free albumin in respiration buffer for isolated mitochondria experiments
- Maintain 2-4% albumin concentration for isolated brown adipocyte studies
- Apply 20mM GDP concentration for tissue sample analysis
Higher GDP concentrations become crucial to inhibit UCP1 in digitonin-permeabilized adipose tissue samples. Notwithstanding that, larger tissue samples might still have active extracellular fatty acids even with 1% albumin concentration.
Quality Control Measures
Quality control steps lead to reliable and repeatable UCP1 research results. Cell Signaling Technology uses strict validation methods:
Multiple validation techniques test antibody specificity. Cross-reactivity analysis proves target selectivity. Strict reproducibility standards ensure consistent results across different test conditions.
Support resources include detailed protocols, troubleshooting guides, and direct access to science teams. Researchers can reach technical support at support@cellsignal.com or call 877-678-8324. These tools help maintain high-quality UCP1 research and solve common lab challenges.
Post-Translational Modifications of Cell Signaling Technology UCP1
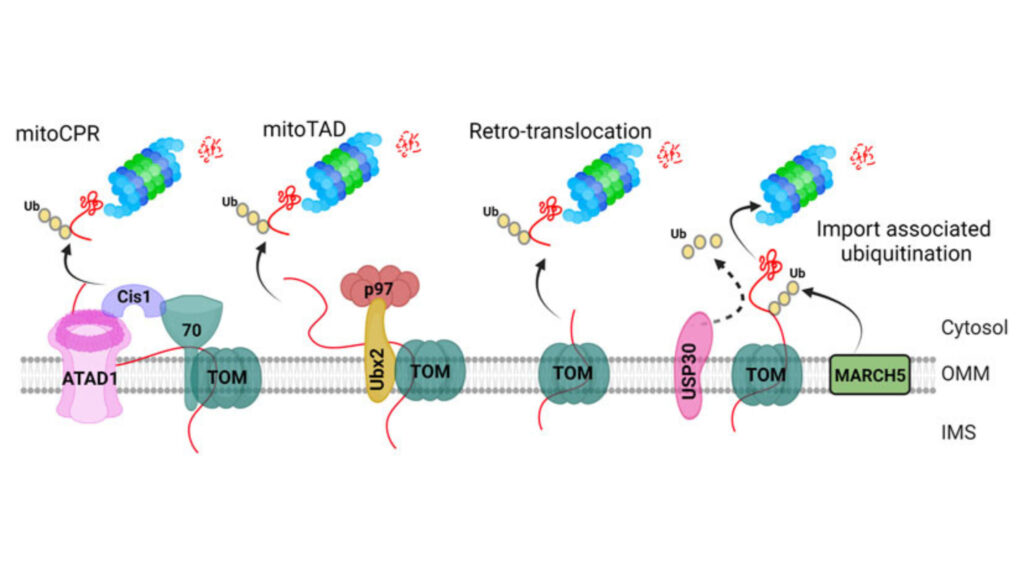
Post-translational modifications (PTMs) shape how UCP1 works and gets regulated in brown adipose tissue. These modifications affect protein stability, activity, and how cells respond to heat production needs.
Phosphorylation Events
UCP1 regulation depends heavily on phosphorylation through complex signaling cascades. The mitochondrial calcium uniporter (MCU) creates a complex with UCP1 that promotes dephosphorylation of PDHE1α at S293. This MCU-EMRE-UCP1 complex, known as the ‘thermoporter,’ helps improve mitochondrial calcium uptake during cold exposure. These phosphorylation events directly affect UCP1’s ability to break down the inner mitochondrial membrane proton gradient.
Ubiquitination Patterns
Ubiquitination is a vital mechanism for UCP1 protein turnover. Studies show that UCP1 goes through ubiquitination both in vitro and in vivo. The cytosolic proteasome breaks down UCP1 at specific rates – taking 30-72 hours in brown adipocytes and about 3 hours in thymocytes. Animals that adapt to cold show higher levels of ubiquitinated UCP1 in their brown adipose tissue mitochondria. MG132, a proteasome inhibitor, stops this breakdown process, while losing mitochondrial membrane potential prevents UCP1 from breaking down.
Cell Signaling Technology UCP1: Protein Stability Control
Several factors affect UCP1’s stability and function. The protein has remarkable structural features:
- Binds three cardiolipin molecules tightly for stability maintenance
- Shows an apparent melting temperature of 27.0 ± 0.7°C without lipids
- Increases stability to 52.8 ± 0.6°C with cardiolipin presence
Succinylation substantially affects UCP1’s stability. When two succinylated lysines change to acyl-mimetic glutamine and glutamic acid, both stability and activity decrease. The acyl-mimetic 2KQ mutant shows a 60% drop in free fatty acid-induced UCP1 activation. GDP binding raises UCP1’s apparent melting temperature by about 24°C and improves stability when lipids are present.
Recent structural analyzes show that GTP takes up the carrier substrate binding site, which prevents activator binding through complex interaction networks that include ionic, polar, and cation-π interactions. While Cys253’s sulfenylation linked to redox activity might boost UCP1 activity, researchers still need to verify the exact mechanism. These modifications work together to keep UCP1 functioning properly and regulated based on heat production needs.
Cell Signaling Technology UCP1: Signaling Pathways in Brown Fat Activation
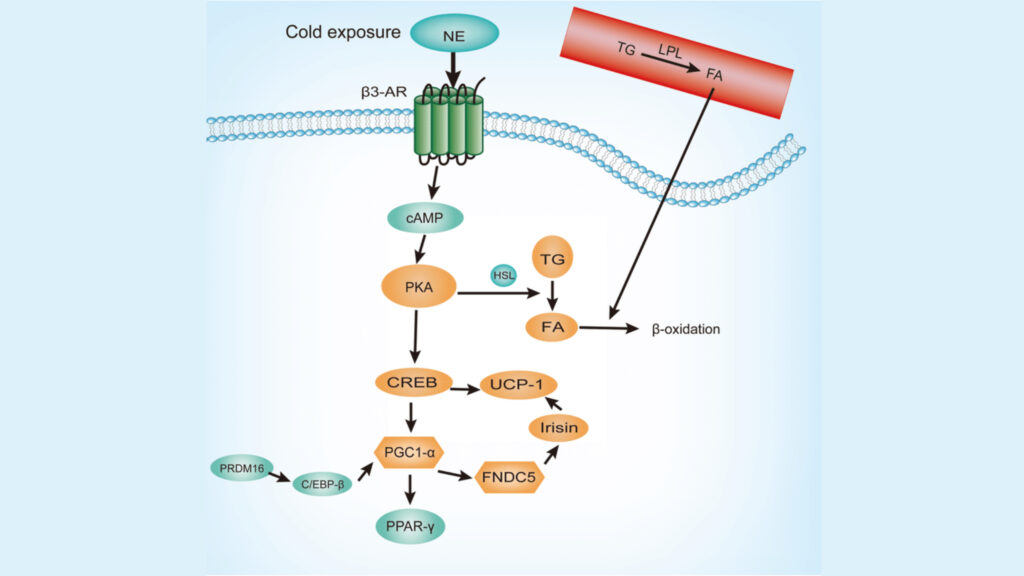
Multiple signaling pathways arrange the complex process of brown fat activation. Each pathway has distinct yet interconnected roles that regulate thermogenesis. These pathways create a complex network that controls UCP1 expression and activity.
MAPK Cascade Involvement
The p38 MAPK pathway is a vital mediator in brown fat thermogenesis. cAMP signaling activates p38 MAPK through PKA in brown adipocytes. p38 MAPK phosphorylates multiple transcription factors, especially ATF2 and PGC1α, which promote UCP1 transcription. Scientists have found that there was ZC3H10 that activates the thermogenic gene program in adipocytes when phosphorylated by p38.
The ASK1-p38 axis responds uniquely to cAMP signaling in adipocytes. Its activation lasts at least 90 minutes and peaks at 15 minutes. This pathway shows tissue-specific regulation, as other cell types show only modest ASK1 response.
PI3K/AKT Signaling
The PI3K-AKT pathway is a vital regulator of brown adipose tissue function. Research shows that insulin signaling positively regulates beige adipogenesis through the PI3K-AKT-UCP1 pathway. The pathway’s core components include:
- Growth factor receptor activation
- PI3K-mediated phosphorylation
- AKT-dependent signaling
- mTORC1 activation
- UCP1 expression regulation
The inhibition of insulin signaling reduces both adipogenesis and white preadipocyte browning. The PI3K/AKT pathway shows substantial upregulation of nerve growth factor receptor and RPS6 expression.
Calcium-Dependent Mechanisms
Calcium signaling adds another layer of complexity to brown fat activation. The sarcoplasmic reticulum (SR) acts as the main cellular calcium store, and SERCA pumps maintain tight regulation. Adrenergic stimulation increases intracellular calcium from about 0.05 μM at baseline to 0.7 μM after stimulation.
The calcium-dependent activation pathway has several key steps. Calcium releases from SR through RyR and IP3 receptors and creates localized increases near mitochondria. Calcium transport into mitochondria through voltage-dependent anion-selective channels and mitochondrial calcium uniporters activates several mitochondrial dehydrogenase enzymes.
Scientists have found that the mitochondrial calcium uniporter forms a complex with UCP1 via EMRE, creating what they call the ‘thermoporter’. This complex helps calcium flow into the mitochondrial matrix, which enhances NADH production and supports thermogenesis in brown adipose tissue.
Cell Signaling Technology UCP1 Validation Methods
Accurate UCP1 research depends on validation methods that need careful experimental design and quality control. Cell Signaling Technology uses strict protocols that will give reliable results for different applications.
Specificity Testing
The acetone precipitation method starts the precise validation of UCP1 detection by removing leftover lipids from tissue lysates. This cleaning method creates clear UCP1 protein bands without any lipid contamination getting in the way. The method works great for white fat depot analysis where lipid parts often make Western blot hard to interpret.
Scientists need to follow these steps to test specificity:
- Acetone precipitation of protein samples
- Double centrifugation of lysates
- Narrow pipette tip extraction under lipid layer
- Loading control verification with actin and HSP90
- Negative control testing using UCP1 knockout samples
Cross-Reactivity Analysis
We tested cross-reactivity to tell UCP1 apart from its homologs. Scientists made monoclonal antibodies using synthetic peptides that match human UCP1’s carboxy terminus. These antibodies don’t react with UCP2 and UCP3 proteins at all. This specificity helps detect the right UCP1 levels in brown and beige adipocytes.
The validation process looks at how antibodies work in human, mouse, and rat samples. Sequence homology analysis suggests these antibodies should work with bovine (93% conserved), canine (93% conserved), and Macaque monkey samples (100% conserved).
Cell Signaling Technology UCP1: Reproducibility Standards
Cell Signaling Technology keeps strict reproducibility standards through detailed validation protocols. The 4C datasets must meet the ‘cis/overall ratio >40%’ standard to show high-quality experiments. The chromatin interactions of UCP1 in iBAT and eWAT reach Pearson correlation coefficients of 0.83 and 0.73. These numbers show great consistency between biological replicates.
Principal component analysis shows clear clustering of chromatin interactions between iBAT and eWAT. Scientists found 368 reliable interaction sites in iBAT and 245 in eWAT through this analysis. These standard methods help get reproducible results in different experimental conditions.
Advanced verification techniques help confirm antibody specificity in the validation process. Each antibody goes through extensive testing with relative expression analysis and cell treatment studies before getting approved for research. The process also checks how well antibodies work in Western Blot, Immunohistochemistry, ELISA, Immunocytochemistry, and Flow Cytometry applications.
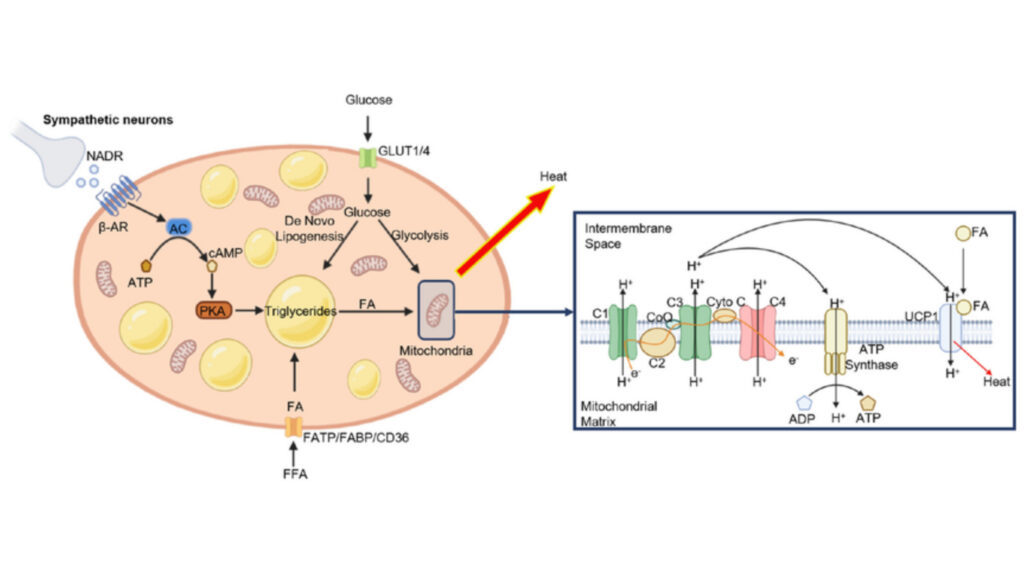
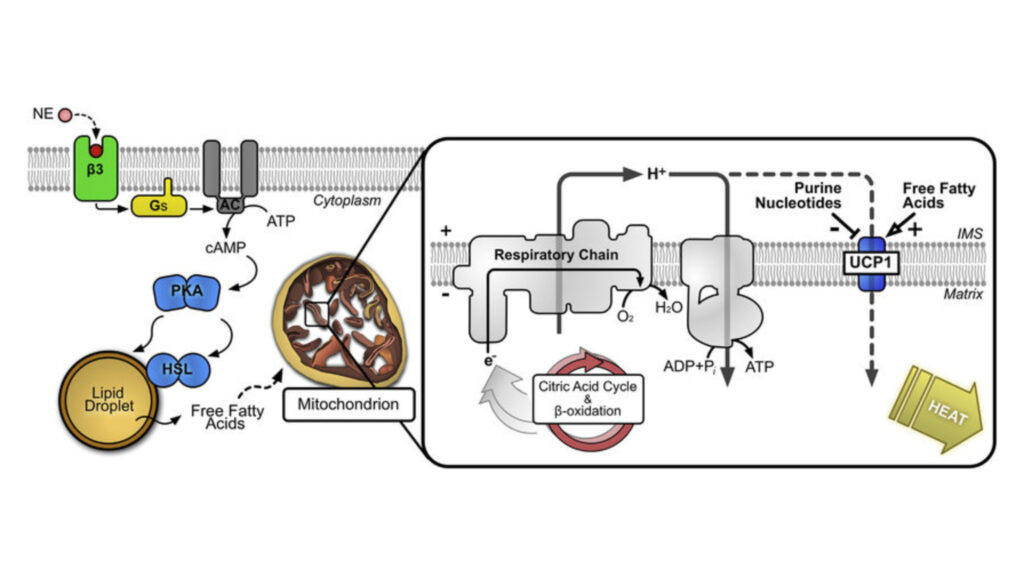
Mitochondrial Regulation by Cell Signaling Technology UCP1

UCP1, a protein in the mitochondrial inner membrane, arranges a sophisticated regulation system that changes cellular energy metabolism. This protein sits in the inner mitochondrial membrane and works as a specialized proton transporter. It dissipates the proton gradient without ATP synthesis.
Proton Leak Mechanisms
UCP1’s proton leak process follows a well-regulated pathway. The protein helps protons re-enter across the mitochondrial inner membrane next to ATP synthase. Long-chain fatty acids are needed as essential cofactors for UCP1 to transport protons. Purine nucleotides naturally inhibit this process by binding to UCP1 with high affinity.
Brown adipose tissue mitoplasts from PSD-iBKO mice show a big drop in total proton current compared to control mice. This difference goes away after adding ATP. We found that mitochondrial phosphatidylethanolamine helps UCP1 activity. This suggests that membrane lipid composition plays a vital role in proton transport.
Electron Transport Chain Interactions
UCP1 creates a unique metabolic environment by interacting with the electron transport chain. The protein’s activation results in:
- Higher oxygen consumption without electron leak
- Better UCP1-dependent respiration
- Changes in oxidative phosphorylation efficiency
- Different proton gradient maintenance
These changes happen without major shifts in electron transport chain enzyme amounts. The lower efficiency of oxidative phosphorylation comes from UCP1’s proton gradient dissipation rather than increased electron leak.
ATP Synthesis Impact
UCP1 changes how cells produce ATP. The activated protein helps protons leak across the mitochondrial inner membrane. This bypasses ATP synthesis and creates heat instead. UCP1 activation causes a 20–35% drop in cellular ATP levels.
The protein’s regulation works through complex molecular interactions. Purine nucleotides bind in UCP1’s central cavity, which keeps the protein open to the cytoplasm. This binding pattern explains why different nucleotides have similar affinities. GTP shows a slightly lower dissociation constant than ATP in radiolabeled nucleotide-binding experiments.
Nucleotide binding depends on pH levels, and binding affinity drops above pH 6.5. This pH sensitivity helps the protein respond to cellular conditions. The binding process involves specific interactions between nucleotides and UCP1. The adenine forms hydrogen bonds with two asparagine residues – N6 bonds with N188 and N3 bonds with N282.
Therapeutic Applications of UCP1 Research
Scientists have made breakthroughs in cell signaling technology that show UCP1 could be a game-changing target for treating metabolic disorders. Research shows UCP1 might help treat obesity and similar conditions because it knows how to control energy use.
Drug Development Strategies
Scientists have changed how they look for UCP1-activating compounds by using high-throughput screening methods. A system using fluorescent cells helped screen FDA-approved drugs and found several compounds that might activate UCP1. The screening platform uses Ucp1–2A-GFP reporter mice, where GFP brightness shows how much UCP1 protein cells make.
Scientists found these promising drug candidates:
- Artemisinin (malaria treatment)
- Resveratrol (cancer therapy)
- Sutent (metabolism modulator)
- Sildenafil (phosphodiesterase inhibitor)
Sutent treatment cut body weight by 16.34% after 16 weeks in mice fed high-fat diets. The treatment helped mice process glucose better and made them more sensitive to insulin. The mice also used more oxygen and produced more CO2, which suggested they burned more energy.
Obesity Treatment Potential
UCP1 activation gives scientists a fresh way to curb obesity because it exists throughout brown fat tissue’s inner mitochondrial membrane. UCP1 stays inactive under normal conditions because purine nucleotides block it. Scientists need to think about several factors when activating it:
Exercise-like compounds such as GW501516 (PPARδ agonist) and β-aminoisobutyric acid (BAIBA) look promising. These compounds boost or copy exercise benefits, though scientists still test how well they work. Thyroid hormone plays a vital part in controlling BAT by raising UCP1 levels.
Naringin shows real promise by boosting UCP1 levels and helping fat metabolism and heat production. Clinical studies suggest that turning on BAT through UCP1 works better than just changing lifestyle habits.
Metabolic Disease Interventions
UCP1 activation helps treat various metabolic disorders. Clinical trials show cold exposure turns on BAT, helps process glucose, and makes diabetic patients more sensitive to insulin. The benefits go beyond just controlling temperature:
- Better glucose processing
- More insulin sensitivity
- More high-density lipoproteins
- Higher plasma bile acids
BAT produces bioactive fats like 12,13-diHOME, 12-HEPE, and Maresin 2 that control fat and glucose metabolism while reducing inflammation in obesity. Scientists used to focus only on temperature control, but new evidence points to broader health benefits.
Treatment needs to target specific tissues to avoid side effects. Scientists could engineer modified fats to turn on UCP1, but making them completely specific remains tough. Understanding key pathways that trigger UCP1 helps develop better treatments.
Looking back at previous studies shows people with more active BAT tend to have lower BMI. Young adults (18-39 years) keep their UCP1 levels and cold-triggered glucose uptake by BAT even when they resist insulin, which suggests treating them early might work best.
Turning on heat production in brown fat tissue could help treat obesity. Finding safe and effective drugs remains a priority. Scientists now work on making chemicals that slightly uncouple breathing in tissues, but they must be careful not to disrupt ATP production in other cells.
Conclusion
UCP1 is a vital regulator of brown fat thermogenesis and shows remarkable potential to treat metabolic disorders. Scientists have uncovered complex molecular mechanisms that govern UCP1 activation and regulation through rigorous research and validation methods.
Western blot protocols and immunofluorescence methods provide precise measurements of UCP1 expression and activity. These advanced detection techniques reveal detailed information about post-translational modifications, signaling cascades, and mitochondrial interactions that control thermogenesis.
UCP1 research has implications beyond scientific understanding. Clinical studies show promising results for obesity treatment through targeted UCP1 activation. New drug development strategies that focus on UCP1-mediated thermogenesis create possibilities to address metabolic diseases.
Scientists are learning about new approaches to control UCP1’s therapeutic potential while ensuring safety and efficacy. Their research indicates that early intervention targeting UCP1 activation might work best, especially in younger adults who maintain active brown adipose tissue despite metabolic challenges.